Our group of "Design and Development of New Antimicrobial Agents" led by Prof. PharmDr. Martin Doležal, Ph D. has recently published an article entitled "Hybridization Approach Toward Novel Antituberculars: Design, Synthesis, And Biological Evaluation of Compounds Combining Pyrazinamide and 4-Aminosalicylic Acid" in the prestigious ACS Infectious Diseases Journal [IF2021 = 5.578 (D1)].
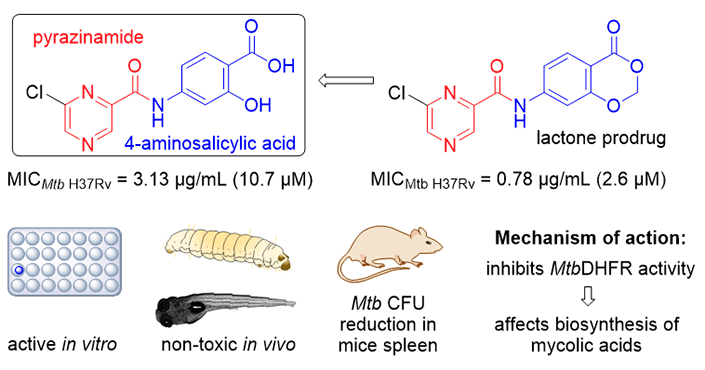
We used a hybridization approach between the first-line antitubercular agent, pyrazinamide, and the second-line antitubercular agent, 4-aminosalicylic acid (PAS), as an attempt to develop novel, potent, antituberculars that solve the fundamental issues of PAS (mainly rapid metabolism and short half-life). Upon several structural manipulations, we were able to identify one promising compound 11 (and its lactone prodrug 11′) with potent, selective, in vitro antimycobacterial activity against both drug-sensitive and drug-resistant strains of Mycobacterium tuberculosis with no in vitro cytotoxicity.
Compound 11 was proven to be non-toxic in vivo in zebrafish and Galleria mellonella models, reduced the number of colony forming units in the murine model of tuberculosis, and targets mycobacterial dihydrofolate reductases. Metabolic studies showed that lactone 11` has t1/2 of 21.4 min in human plasma, and is opened to compound 11 that is in return metabolized by human liver microsomes (t1/2 = 187 min). The long half-life of compound 11 overcomes the main drawback of PAS, that is, frequent dosing due to its short half-life.
This work is a presentation of medicinal chemistry in its interdisciplinarity, including drug design, synthesis, in vitro and in vivo studies, metabolic experiments, and determination of mechanism action. It also shows the importance of scientific collaboration since our work is the result of combined efforts of our institutional (Pharmaceutical Analysis Group; Experimental Toxicokinetics and Radiopharmacology), national (Department of Clinical Microbiology, University Hospital; Biomedical Research Center, University Hospital Hradec Kralove; Military Health Institute, Military Medical Agency, Prague), and international (Department of Biosciences, University of Oslo; Department of Biochemistry, Faculty of Natural Sciences, Comenius University in Bratislava) collaborators.
The study was supported by EFSA-CDN (No. CZ.02.1.01/0.0/0.0/16_019/0000841) co-funded by ERDF, and by the Czech Science Foundation project No. 20-19638Y. Supported by Ministry of Health of the Czech Republic, grant no. NU21-05-00482 (grant to J.Z.). This study was partially supported by MH CZ-DRO (University Hospital Hradec Kralove, Nr. 00179906), by Charles University, Czech Republic (Nr. SVV 260 547), by Slovak Research and Development Agency (grant n. APVV-19-0189), and by the OPII, ACCORD, ITMS2014+: 313021X329, co-financed by ERDF.
Text: Ghada Bouz, Ph.D. and Assoc. Prof. PharmDr. Jan Zitko, Ph.D.